Genetically Modified (GM) crops
2021 NOV 8
Mains >
Agriculture > Crops > Genetic engineering
IN NEWS:
- Since June, the export of about 500 tonnes of rice from India has triggered an uproar in several European countries on the grounds that it was genetically modified (GM) rice.
GENETICALLY MODIFIED (GM) CROPS:
- Genetic modification of plants involves adding a specific stretch of DNA into the plant’s genome, giving it new or different characteristics. This could include changing the way the plant grows, or making it resistant to a particular disease.
- Engineered genes are added or removed using genetic engineering techniques such as gene guns, electroporation, microinjection, agrobacterium, CRISPR and TALEN.
- The technology is also called “modern biotechnology” or “gene technology”, “recombinant DNA technology” or “genetic engineering”.
- The first genetically engineered crop plant was tobacco, reported in 1983. However, the first commercialized GM crop was “Flavr Savr” tomato, engineered to have long shelf-life.
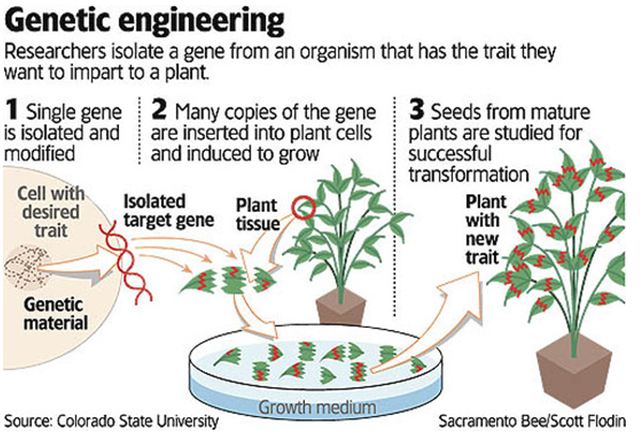
TYPES OF MODIFICATION:
- Transgenic: The genetic modification of a recipient plant with one or more genes from any non-plant organism. The inserted genes can come from species within the same kingdom (plant to plant), or between kingdoms (for example, bacteria to plant).
- Cisgenic: The genetic modification of a recipient plant using genes found within the same species or a closely related one.
- Intragenic: refers to the transference of new combinations of genes and regulatory sequences belonging to that particular species.
- Subgenic: Altering the genetic makeup of a plant without incorporating genes from other plants, by deleting part of the gene.
INTERNATIONAL REGULATORY EFFORTS:
I. UN CONVENTION ON BIOLOGICAL DIVERSITY:
- It is a multilateral legally-binding treaty that finds its origin at the Earth Summit in Rio De Janeiro in 1992.
- It has 3 main objectives:
- The conservation of biological diversity
- The sustainable use of the components of biological diversity
- The fair and equitable sharing of the benefits arising out of the utilization of genetic resources
- The Biological Diversity Act, 2002 was enacted for giving effect to the provisions of the Convention.
II. CARTAGENA PROTOCOL ON BIOSAFETY:
- Cartagena Protocol on Biosafety is a supplementary agreement to the UN CBD.
- The international legally binding treaty concerns the movement of LMOs (living modified organisms) resulting from modern technology from one nation to another. It seeks to protect biodiversity from the potential risks caused by LMOs arising from modern technology.
- The protocol was adopted in Montreal in 2000 but is named after Cartagena, the original city in Colombia where the protocol was supposed to be adopted.
- The Protocol has provisions for:
- An Advance Informed Agreement (AIA) procedure to ensure that countries are given enough information to make informed decisions before agreeing to import LMOs into their country.
- A Biosafety Clearing-House (BCH) to enable information exchange on LMOs between countries.
- India ratified the protocol in 2003. The nodal agency for the implementation of the Protocol is the Ministry of Environment, Forest and Climate Change (MOEF&CC).
III. NAGOYA PROTOCOL:
- The Nagoya Protocol on Access to Genetic Resources and the Fair and Equitable Sharing of Benefits Arising from their Utilization (ABS) is also a supplementary to the UN CBD.
- The objective of this international legally binding protocol is the fair and equitable sharing of benefits coming from the utilization of genetic resources and helping in the conservation & sustainable usage of biodiversity.
- India signed the Protocol in 2011 and ratified it in October 2012.
GM CROPS IN INDIA:
- The only approved GM crop in India till date is BT Cotton, introduced in 2002.
- The area under Bt. Cotton has increased from 0.29 lakh hectares in 2002-03 to 117.47 lakh hectares in 2019-20, which is almost 94% total area under cotton cultivation in India.
- In 2010, Bt brinjal was cleared for commercial cultivation, but the environment ministry later placed an indefinite moratorium on it. Similarly, the approval of commercial cultivation of GM mustard was withdrawn in 2017.
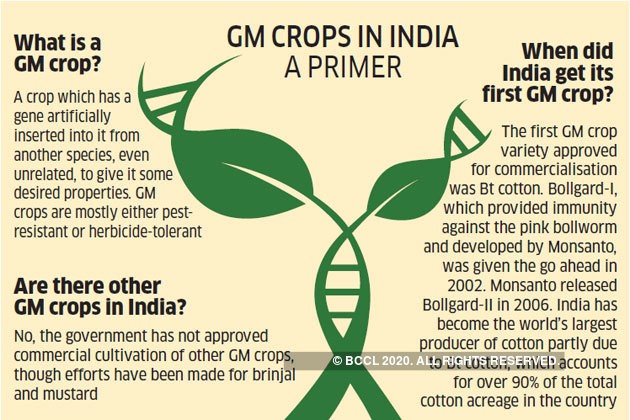
NATIONAL REGULATORY EFFORTS:
I. ENVIRONMENT (PROTECTION) ACT, 1986
- Genetically modified organisms (GMOs) and crops are regulated under the Environment (Protection) Act, 1986 and rules notified under it.
- Under the Act, planting unapproved GM seed varieties can attract a five-year jail term and a fine of up to Rs. 1 lakh.
II. GENETIC ENGINEERING APPRAISAL COMMITTEE (GEAC):
- The GEAC is India’s apex biotechnology regulatory body. It functions under the Ministry of Environment, Forest and climate change.
- It is chaired by the Special Secretary/Additional Secretary of MoEF&CC and co-chaired by a representative from the Department of Biotechnology (DBT).
- It accords environmental approval of activities involving large scale use of hazardous microorganisms and recombinants in research and industrial production.
- It is also mandated with approving the release of genetically engineered organisms and products into the environment, including experimental field trials.
III. REVIEW COMMITTEE ON GENETIC MANIPULATION (RCGM):
- It functions under the Department of Biotechnology (DBT), Ministry of Science and Technology.
- It is mandated with monitoring and regulating safety related aspects of ongoing research projects and activities, including small scale field trials.
IV. BIOLOGICAL DIVERSITY ACT, 2002:
India enacted Biological Diversity Act in 2002 for giving effect to the provisions of the CBD. The Act envisages a three-tier structure to regulate the access to biological resources:
- National Biodiversity Authority (NBA) at the central level:
- It performs facilitative, regulatory and advisory function for the Government of India on issues of conservation, sustainable use of biological resources and fair and equitable sharing of benefits arising out of the use of biological resources.
- A foreign entity needs to take the permission from the NBA for utilizing the biological resources or accessing the knowledge thereof, for a survey, research, and commercial purposes.
- State Biodiversity Boards (SBB) at the state level:
- They advise the state government on matter related to biodiversity.
- Indian nationals and companies need to take the permission from SBBs to utilize the country's biological resources.
- Biodiversity Management Committees at the local level:
- They are tasked with preserving and protecting the local biodiversity. They are mandated to prepare a Peoples' Biodiversity Register (PBR) in consultation with the local people.
- They have the right to levy fees on any individual or company for accessing and utilizing the local biodiversity or the knowledge thereof.
V. Other Acts and rules concerned with GM Crops:
- Rules for The Manufacture, Use/Import/Export and Storage of Hazardous Micro Organisms/ Genetically Engineered Organisms or Cells (Referred to as Rules 1989)
- Plant Quarantine (Regulation of Import into India) Order, 2003
- Food Safety and Standards Act, 2006
PROCEDURE FOR APPROVAL OF GMOs:
- The company involved in the development of the GM crop undertakes several biosafety assessments including environmental safety, food and feed safety assessments in containment.
- This is followed by Bio-safety Research Trials in two stages-Biosafety Research Level-(BRL) trial I and BRL-II, which require prior approval of RCGM and GEAC respectively.
- Approval for environmental release is accorded by the GEAC after it considers the findings of the bio-safety and agronomic studies as well as recommendations of the RCGM and other committees.
- Finally, commercial release is permitted by the GEAC for only those transgenic crops that are found to be safe for humans and the environment.
SHOULD INDIA PURSUE GM CROPS:
YES:
- Promising results:
- Eight years after the deployment of Bt cotton, India became the top exporter of cotton globally and the second largest cotton producer in the world.
- Also, Bangladesh has been successfully cultivating the Bt Brinjal developed by an Indian firm for several years.
- Address food security and hidden hunger:
- Genetic modification can increase both quantity as well as the nutritional value of foods. Such technological advances can save millions from starvation and poverty.
- For example, rice with high beta carotene, also called golden rice, was developed to address deficiency in vitamin A.
- Improve farmers’ income:
- GM crops offers exponential rise in production. This, coupled with increased resistance to failures and reduced costs of inputs such as pesticides can greatly improve the income from farm fields. This will, in turn, have a positive effect on India’s overall economy.
- Environmental benefits:
- Reduced use of chemical pesticides and insecticides can reduce environmental pollution and improve the quality of products.
- For instance, a normal brinjal crop requires up to 30 sprays of insecticides. But a Bt variety requires fewer sprays.
- Economic benefits:
- Use of genetically modified corn, cottonseed, soybean and potato as livestock feed can increase the productivity from livestock. Also, the use of GM oilseeds can drastically reduce India’s import dependency on edible oil.
- Meet a climate uncertain future:
- GM crops can be engineered to withstand weather fluctuations and extremes that India may face due to climate change. Eg: Salt tolerant transgenic rice variety, which was developed by Bose Institute in Kolkata.
- Indigenous development capacity:
- India is steadily moving towards self-sufficiency in developing indigenous GM crops and reducing dependency on multinational corporates. Bt Brinjal and Dhara Mustard Hybrid-11 (DMH-11) are examples.
NO:
- Regulatory flaws:
- For approval in India, most of the tests are conducted by the company itself. This raises questions over the reliability of tests.
- Also, standing committee on Agriculture in 2012 found that in both Bt cotton and Bt brinjal, the requisite numbers of tests were not carried out in the country.
- Miscalculations:
- Critics argue that the rise in cotton yields can be explained by improvements in irrigation and a dramatic growth in the use of fertilizers and not solely due to the GM crop.
- Unsustainable:
- In areas of BT cotton cultivation, initial production was high given that the crop was pest resistant. However, eventually small and marginal farmers suffered losses because of high input costs and yield loss due to the development of resistance in the targeted pests such as the pink bollworm.
- Corporate monopoly:
- Patent laws give developers of the GM crops, such as Monsanto, monopoly over the supply of seeds, resulting in fewer choices and higher cost for seeds.
- Eg: Monsanto’s "terminator seeds" are modified seeds that are designed to only last one generation. This is to ensure that farmers have to annually purchase new seeds from the organization.
- Eliminates local varieties:
- Excessive use of GM varieties have resulted in the near-complete destruction of indigenous crop varieties and diminished the genetic diversity. Eg: The cultivation of Bt cotton has caused traditional non-GE cotton varieties to be wiped out, thereby reducing farmer seed choices.
- Indirectly leads to farmer suicides:
- The high input costs, combined with lack of traditional varieties, indebtedness and resurgence of pests were the root causes of farmer suicides in the country.
- Impact on health:
- GM Crops such as Bt brinjal poses risks to human health, as it is resistance to antibiotics and can turn medicines ineffective in the future. Also, Herbicide-tolerant GM crops have led to an increase in the use of herbicides such as Glyphosate. Its rampant use has increased the risk of cancers and other health issues.
- Environmental cost:
- When the GM crops breed with non-GE and wild relatives, it can result in genetic contamination, resulting in far-reaching environmental impact.
- Eg: In the US, Monarch butterflies and their principal host plant, the milkweed, have been pushed to near extinction after the introduction of GM corn.
OTHER CHALLENGES/CONCERNS:
- Illegal use:
- In spite of the ban, GM seeds are available in the black market and farmers are already cultivating them. There were also reports of illegal field trials being conducted throughout the country, which has potentially exposed farmers and consumers to untested technology.
- Negative public perception:
- Regulatory failures and fear of seed monopoly has created a deep distrust among the common masses regarding GM crops.
- States are ignored:
- State governments are not mandatorily consulted for conducting open field trials on GM crops. Several states such as Kerala and Bihar have opposed field trials for GM crops.
- Poor awareness:
- Farmers and consumers have little avenue to identify a GM crop/product and make an informed choice. While FSSAI mandates labelling of packaged food products with over 1 % of GM ingredients, it is difficult to implement as most products in India are sold unpacked in an open manner.
- Small land holdings:
- India’s small and marginal holdings (below two hectares) constituted 86.21% of the total land holdings. For them, GM crop cultivation in the long run will be unaffordable, given the high input costs.
- Issues within GEAC:
- The GEAC has no statutory backing or representation from civil society. After approving the crop, it is also responsible for evaluating its own decision to approve the crop. This leads to a conflict of interest. It also lacks transparency, as it rarely releases the data on various safety tests.
ILLEGAL CULTIVATION OF GM CROPS:
Despite the risk of prosecution, farmers in India have been found growing illegal HT cotton and BT Brinjal, which have not been cleared for commercial cultivation. This situation arises mainly because of:
- Easy availability:
- Some GM unapproved varieties in India are already being cultivated by its neighbours, like Bangladesh cultivating BT Brinjal. These eventually reach the Indian farmers either illegally or unknowingly.
- Lack of awareness:
- Farmers have no means to distinguish between GM and non-GM crops.
- Support from influential groups:
- Several influential agrarian groups like Shetkari Sangathana in Maharashtra have announced agitation for use of unapproved genetically modified seeds and has even called for open defiance of restrictions.
- Rising cost of agriculture:
- Cost of cultivation, particularly farm labour cost has increased in India, making farmers move towards GM crops.
- Eg: HT cotton, grown globally since 1995, is designed to withstand herbicides, which kills weeds but leaves the plant unaffected. This results in significant savings on labour costs.
- Increasing risk in agriculture:
- Climatic variations resulting in frequent droughts and floods have increased the risk of agriculture in India. Hence farmers are looking of climate resilient variants offered by genetic modifications.
- Failure of regulatory mechanisms:
- The continued cultivation of GM crops highlight the fact that regulatory bodies have failed to enforce the legislations banning the cultivation of GM crops in the country.
WAY FORWARD:
- Biotechnology Regulatory Authority of India:
- There is a need for an independent authority to ensure that biotechnology is introduced without compromising the safety of biodiversity, human health, and environmental protection. For this, the Biotechnology Regulatory Authority of India Bill, 2013 needs to be revisited.
- Strengthen regulatory framework:
- The regulatory framework should be given statutory backing to ensure strong oversight over GM organisms. Its composition should also be altered to include various stakeholders.
- Increase transparency:
- Effective discussion with scientific community, civil society and state governments etc. should be made mandatory in the process of introducing GM crops. The reports and results of tests should be made available to the public.
- Improve accountability:
- There should be a proactive patent regime, strong liability laws and accountability mechanisms to prevent monopolization and ensure that developers act responsibly while introducing GM crops. It will also help improve public trust towards GM technology.
- Separation of functions:
- The evaluation of reports on GM crops should be entrusted with an agency other than the GEAC, such as the Council of Scientific and Industrial Research to prevent conflict of interest.
- Prioritise local varieties:
- Government backing for resources, infrastructure and seeds is essential to scale up ‘desi’ varieties.
- Improve awareness:
- The government should strengthen the labelling of GM products so that consumers are able to make informed choices.
PRACTICE QUESTION:
Q. ‘Genetically Modified (GM) crops can become the driver of next green revolution in India’. Critically analyse?

