India’s Vaccine Industry
2020 AUG 17
Mains >
Science and Technology > Bio technology > Basics of biotechnology
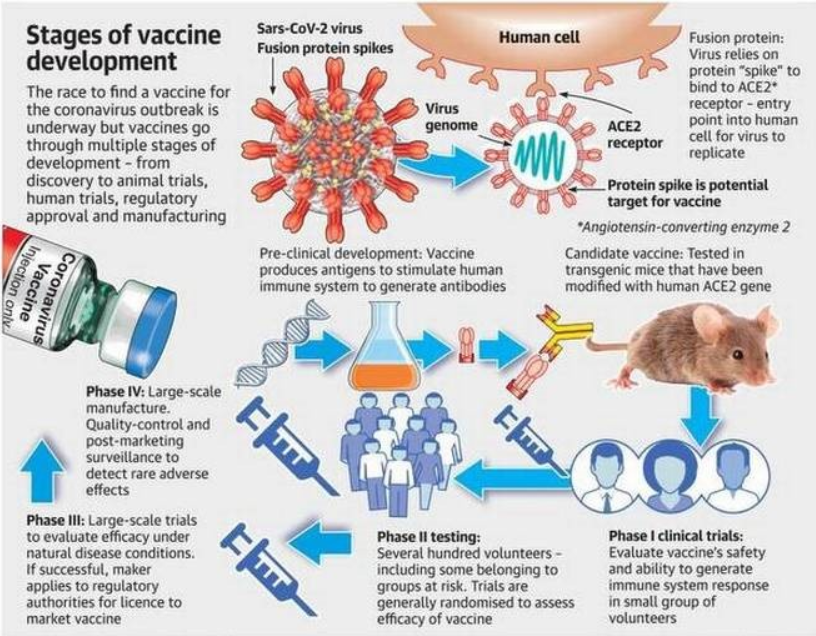
IN NEWS:
- Russia became the first country to officially register a coronavirus vaccine dubbed ‘Sputnik V’ and declare it ready for use. Besides this, over 40 different candidate vaccines for COVID-19, including India’s ‘Covaxin’ are in development.
WHAT IS VACCINE:
- A vaccine is a type of preventive medicine that trains the body’s immune system so that it can recognise and fight a pathogen it has not come into contact with before.
- A vaccine contains certain molecules from the pathogen, called antigens, that resembles a disease-causing microorganism and trigger an immune response from the body.
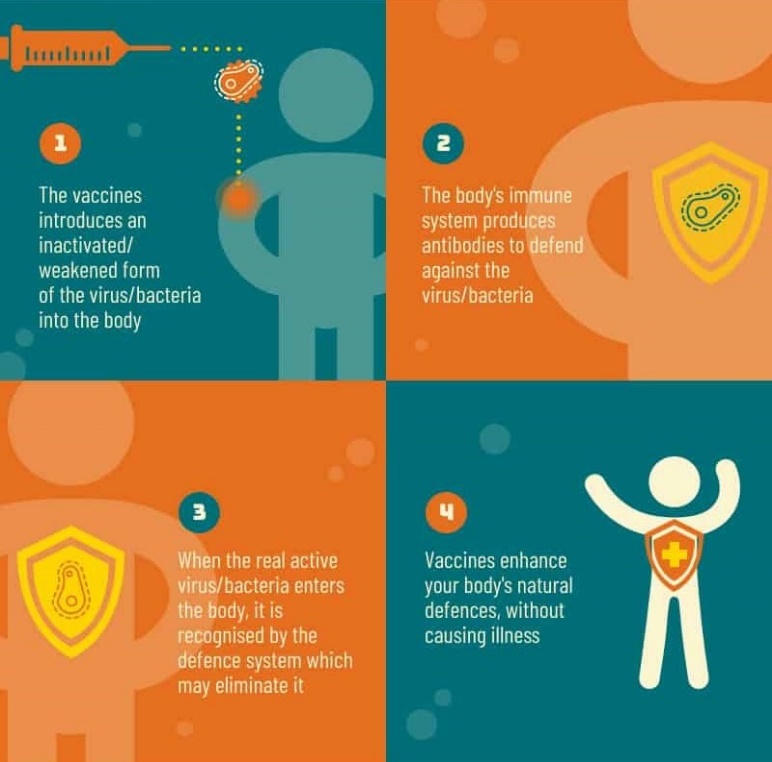
- The antigen is often made from weakened or killed forms of the microbe, its toxins, or one of its surface proteins.
- Inactivated: These vaccines contain inactivated, but previously virulent micro-organisms that have been destroyed with chemicals, heat, or radiation. Examples include the polio vaccine and rabies vaccines.
- Attenuated: These vaccines contain live, attenuated microorganisms. These are active viruses that have been cultivated under conditions that disable their virulent properties. Examples include the BCG vaccine against tuberculosis and measles and rubella vaccines.
- Toxoid: These vaccines are made from inactivated toxic compounds that cause illness rather than the micro-organism. Examples of toxoid-based vaccines include tetanus and diphtheria.
- Subunit, recombinant, polysaccharide, and conjugate vaccines: These vaccines use a specific piece of the germ — like its protein, sugar. These vaccines are used against Hepatitis B and whooping cough.
- Edward Jenner is considered the founder of vaccinology, for his contributions towards the development of smallpox vaccine.
- Vaccines don't just work on an individual level but protect entire populations through "herd immunity" or "community immunity".
STAGES OF DEVELOPING A VACCINE:
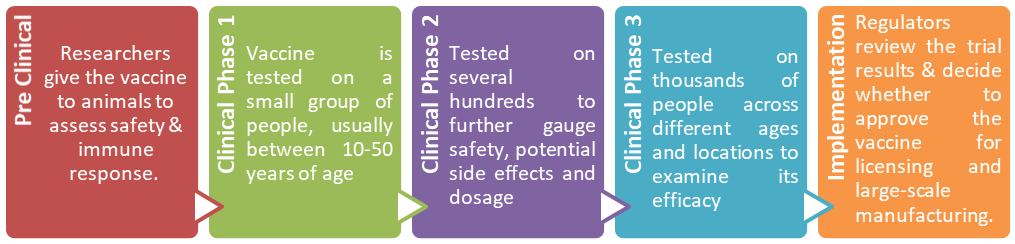
- A vaccine must go through multiple stages before being green lit for use. Typically, each step can take two years or more to complete.
- But in the race to stop the coronavirus, some of those steps are being combined, or skipped altogether to speed up the process.
INDIA’S VACCINATION EFFORTS:
- Vaccination is a major part of the public health policy of independent India. India has eradicated diseases such as smallpox and polio, based on dedicated governmental efforts such as the Universal Immunisation Programme and National Vaccine Policy.
Universal Immunization Programme:
- UIP is one of the largest public health programmes targeting close of 2.67 crore newborns and 2.9 crore pregnant women annually.
- Under UIP, immunization is providing free of cost against 12 vaccine preventable diseases:
- Nationally against 9 diseases - Diphtheria, Pertussis, Tetanus, Polio, Measles, Rubella, severe form of Childhood Tuberculosis, Hepatitis B and Meningitis & Pneumonia caused by Hemophilus Influenza type B
- Sub-nationally against 3 diseases - Rotavirus diarrhoea, Pneumococcal Pneumonia and Japanese Encephalitis.
- The two major milestones of UIP have been the elimination of polio in 2014 and maternal and neonatal tetanus elimination in 2015.
National Vaccine Policy:
- Released in 2011, the policy document addresses broad issues to strengthen Universal Immunization Programme in India and streamline the decision-making process on new and underutilized vaccine introduction.
- The document also addresses the issues of vaccine-security, management, regulatory guidelines, vaccine research & development and product development.
Mission Indradhanush:
- Mission Indradhanush (MI) was launched in December 2014 and aims at increasing the full immunization coverage to children to 90%.
- Under this drive, focus is given on pockets of low immunization coverage and hard to reach areas where the proportion of unvaccinated and partially vaccinated children is highest.
Electronic Vaccine Intelligence Network (eVIN) rollout:
- The Government has rolled out the eVIN system that digitizes the entire vaccine stock management, their logistics and temperature tracking at all levels of vaccine storage – from national to the sub-district.
- This enables program managers to have real time view of the vaccine stock position and their storage temperature across all the cold chain points providing a detailed overview of the vaccine cold chain logistics system across the entire country.
INDIA’S VACCINE INDUSTRY:
- Over the years, India has emerged as the global vaccine hub. The Indian market size was Rs 94 billion in 2019 and is expected to reach a valuation of Rs 252 billion by 2025.
- Indian vaccine industry has the largest global capacity for the manufacture of cost-effective vaccines. It solely accounts for around 60% of the total vaccines supplied to the UNICEF.
- The Indian vaccine industry began as a network of state-owned manufacturers supplying basic pediatric vaccines to the national immunization program. In recent decades, the number of private owned firms active in the sector has grown rapidly.
- Key drivers for the vaccine market in India are:
- Relatively low cost of manufacturing
- Promising Research & Development institutions
- Low cost of clinical trials
- Availability of skilled manpower and scientists
- A large, local consumer base
- Assistance from Government bodies like the Department of Biotechnology and the Indian Council of Medical Research
- Export oriented production
- Major vaccine developers in India include Serum Institute of India Limited, Shantha Biotechnics, Bharat Biotech Limited and Indian Immunologicals Limited.
CHALLENGES TO INDIAN VACCINE INDUSTRIES:
- High capital investment: Vaccine manufacturing involves huge capital investment upfront, which only large corporates and state-owned enterprises can afford. Both technology and capital requirements are significant barriers to entry for startups in the segment.
- Complex regulatory mechanism: Regulatory pathway for vaccines is currently laden with delays and redundancies. There are multiple agencies involved in the regulatory process and the overall timeline for product approval is much stretched.
- Data deficiency: Getting quality epidemiological and surveillance data is a challenge. Accurate incidence reporting and burden of disease estimates are all in infancy. This affects effective researches as well as post-marketing surveillance of vaccines.
- Limited diversity: The Indian vaccine industry has for years focused on improving generic vaccines, rather than develop vaccines for newer diseases such as Zika.
- Intellectual Property regime: The IP regime in India, particularly in pharmaceutical sector, has been viewed in bad light for a long time, owing to the compulsory licensing regime and government support for generic medicines.
- Lack of sufficient cold chain infrastructure: The supply chain of vaccines requires strong quality control systems such as cold storages, as the vaccines tend to be very sensitive to temperature variations. However, logistical challenges exist in India due to its under-developed supply chain system.
- Human resource: Getting industry ready candidates from educational institutions is a challenge. Most candidates have limited practical knowledge and companies are forced to spend on training them.
- Access to latest technologies: Indian vaccine industry has for years lagged behind the western world owing to lack of access to state-of-the-art technologies and equipment. However, the FDI rules are still evolving, slowing giving the industry the much-needed access to latest technologies and equipment.
WAY FORWARD:
The vaccine for Covid-19 may be developed anywhere in the world, but the production of required quantities may not be feasible without the involvement of Indian manufacturers. However, India can leverage on this advantage only if it takes proactive measures:
- Become self-reliant: Currently, 80% of the Indian government’s need for vaccines is met by private firms in India and abroad. India has to empower its public sector vaccine industries to ensure cost-effective and dependable manufacturing of vaccines to meet its needs.
- Streamline regulatory framework: The number of agencies involved in approval and oversight should be streamlined in order to avoid delays and redundancies in vaccine development.
- Ensure reliability: By cutting red tape, procedures can be prioritised and sped up, but they must not be skipped. The Vaccine Regulatory Authority of India must ensure that all safety standards and ethical testing practices are adhered to.
- Develop enabling ecosystem: Augmenting research, production and logistics capacities, international academic collaborations and improving financial allocation for public health infrastructure will go a long way to create an enabling ecosystem for vaccine development in the country.
- Develop central database: India must develop an accurate directory covering various aspects such as disease burden, healthcare providers & facilities, details of vaccine trials etc.
- Prevent vaccine nationalism: Global public goods such as vaccines should not be hijacked by wealthy nations. To prevent vaccine nationalism and ensure equitable access, India has to utilise its ties with other governments and multilateral forums such as the UN and WHO.
PRACTICE QUESTION:
Q. India’s vaccine manufacturing capabilities is positioned to play a crucial role in the global eradication of COVID-19. Examine?


